Beyond the Refrigerator: Integrating VVM Cold Chain and Medical Cold Chain Boxes into Your Compliance System
Introduction: Compliance—The Lifeline of Your Cold Chain
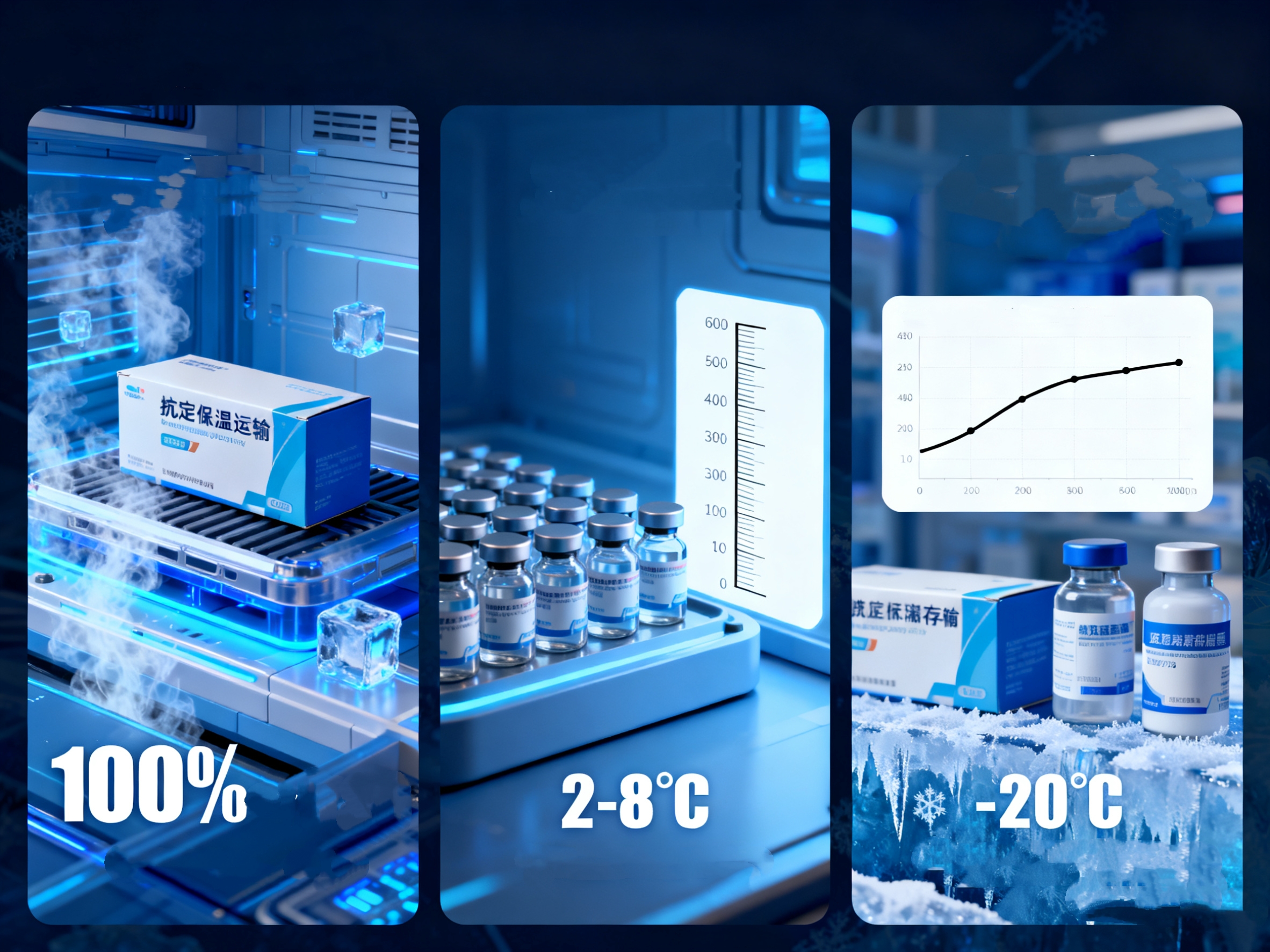
In the world of Vaccine Logistics and Cold Chain Management, compliance is not optional—it is mandatory. We understand that as a facility manager or compliance officer, you need to do more than just keep vaccines cold; you need to provide irrefutable evidence that they stayed cold.
Many facilities still treat the VVM cold chain (Vaccine Vial Monitor) as their primary line of defense. However, a VVM is merely a passive indicator—it tells you when you have already lost a shipment.
We provide
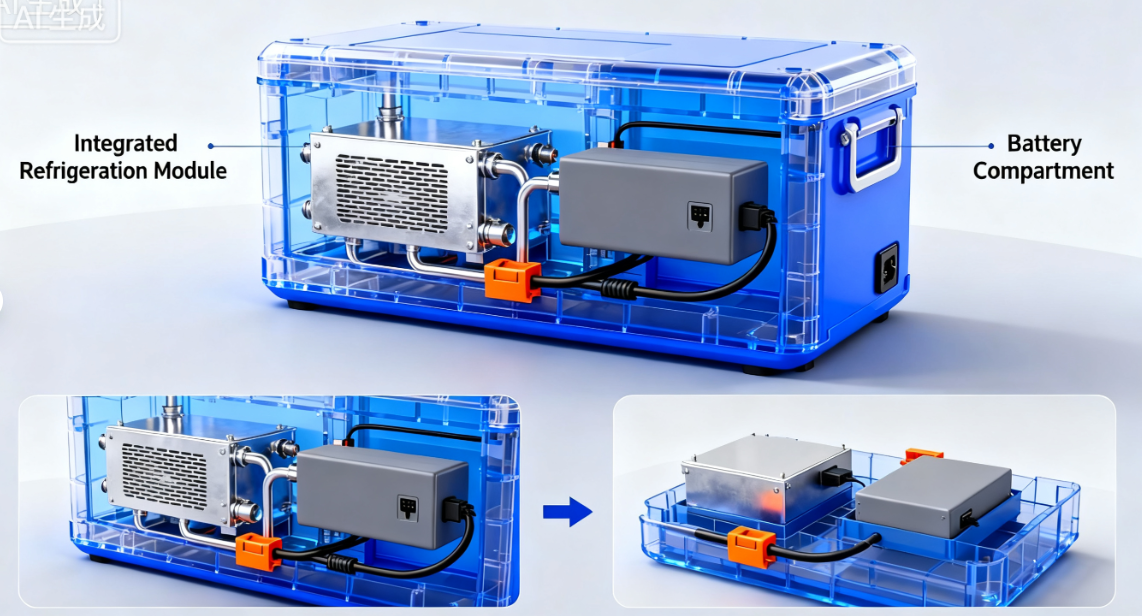
Medical Cold Chain Boxes designed to resolve this fundamental conflict, upgrading your management strategy from reactive loss confirmation to proactive prevention and real-time intervention. Below, we demonstrate how integrating our high-precision active equipment into your Cold Chain System in Immunization ensures comprehensive compliance.
Indicator vs. Solution: The Fundamental Limitation of VVM
We must draw a sharp distinction between the role of a VVM and Active Control. A VVM sticker changes color to tell you if a vaccine has been exposed to dangerous heat, at which point you must discard it. It is an autopsy tool, not a life-support system.
Our Medical Cold Chain Boxes are designed to prevent the damage from happening in the first place.
| Feature Comparison | VVM Cold Chain (The Indicator) | Our Active Medical Cold Chain Boxes (The Solution) |
| Functional Role | Reactive Indicator: Confirms damage after it happens. | Preventative System: Actively maintains safety. |
| Control Capability | Zero control. Cannot stop temperature excursions. | Active Cooling/Heating: Precisely maintains target temperature via compressor. |
| Data Limitations | Only records cumulative heat exposure. No time/location logs. | Full Data Logging: Records time, GPS location, and granular temperature trends. |
| Outcome | Results in vaccine wastage/disposal. | Intervention Possible: Prevents wastage before it occurs. |
A true Vaccine Storage Solution must be active. While VVMs are a necessary part of the audit trail, our equipment is the core safeguard ensuring vaccine potency.
Meeting Formal Storage Requirements: Safety in the Details
Pharmacy Vaccine Storage regulations are stringent. We have engineered our equipment to meet and exceed global health standards (such as WHO/CDC guidelines) for mobile storage units in three key areas:
Data Integrity and Audit Readiness
Immutable Records: Our units feature built-in high-precision sensors and data loggers. All data—including temperature fluctuations, timestamps, and GPS coordinates—is stored in a format that cannot be altered, ensuring you are always audit-ready. This drastically simplifies the record-keeping process for your Vaccine Logistics and Cold Chain Management.
Automated GSP Reporting: Our system can automatically generate GSP-standard temperature control reports. This feature alone can reduce audit preparation time by up to 85%, freeing your staff from manual data entry.
Hygiene Standards in Medical Environments
Sanitation is critical. Unlike traditional metal or porous foam containers, our device shells are constructed from High-Density Polyethylene (HDPE).
Sanitation Ready: HDPE is corrosion-resistant, non-toxic, and features a smooth surface that is easy to clean. This allows for high-frequency disinfection required in medical settings without degrading the equipment.
System Integration: Turning Data into Compliance
Raw temperature data has limited value unless it is integrated into your management platform. Our active containers serve as the intelligent data entry point for your Cold Chain System in Immunization.
Real-Time Monitoring and Pre-emptive Alerts
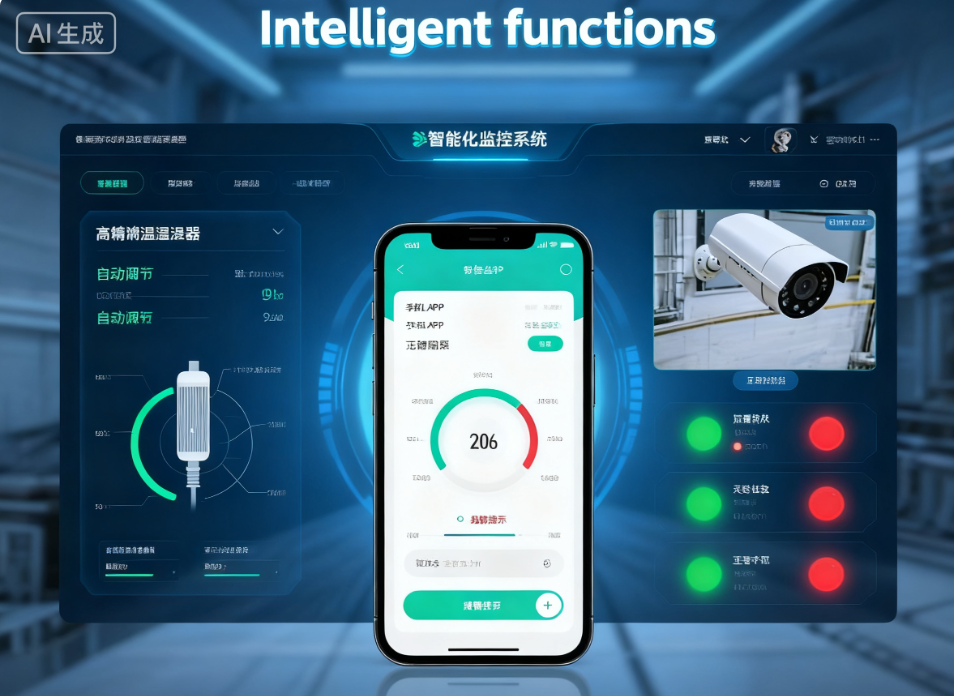
Through IoT and GPS technology, our containers transmit real-time humidity, temperature, and location data to the cloud.
Visualized Management: You can track the exact status of your assets anywhere in the world.
Remote Intervention: If the internal temperature shows a trend toward deviating from the safe range, our system triggers an immediate automatic alarm. This allows compliance managers to intervene remotely via App or PC, adjusting settings or rerouting shipments before a violation occurs.
Strategic Positioning: The Ultimate Backup and Mobile Solution
We position our Medical Cold Chain Boxes as flexible, high-precision assets that extend compliance beyond the walls of your fixed
Pharmacy Vaccine Storage.
The 72-Hour "Safety Net" for Power Outages
In the event of a facility power failure or a breakdown of your main pharmaceutical fridge, our units act as the ultimate emergency backup.
Extended Autonomy: Thanks to a massive 6400Wh battery capacity, our units can maintain freezing temperatures (e.g., -18°C) for 24 hours in 30°C heat.
Emergency Resilience: In sudden power loss scenarios, our insulation and battery combination provides up to 72 hours of continued operation. This capability significantly reduces the risk of catastrophic inventory loss and can reduce related insurance claims by 90%.
Conclusion
In the realm of
Vaccine Storage Solutions, proactive prevention is infinitely superior to reactive confirmation. Our Medical Cold Chain Boxes offer the military-grade durability, high-precision active control, and seamless data integration necessary to build a modern, audit-proof Cold Chain System in Immunization.
Don't just measure the loss—prevent it.
Do not settle for VVM indicators that only tell you when it's too late. Contact our compliance experts today to learn how to integrate our intelligent Medical Cold Chain Boxes into your Vaccine Logistics and Cold Chain Management platform.
Mobile Phone :+86 15890051653
Email :melissa@newbasen.com
Website :https://www.newbasecool.com